Whether you are within your product innovation phase, currently planning the development or determining an approval strategy: the correct classification of a medical device is important - but sometimes also tricky.
Our experts are familiar with all specialities in medical device development and have concentrated and combined their knowledge in this classification tool. We are pleased to provide this tool for you free of charge.
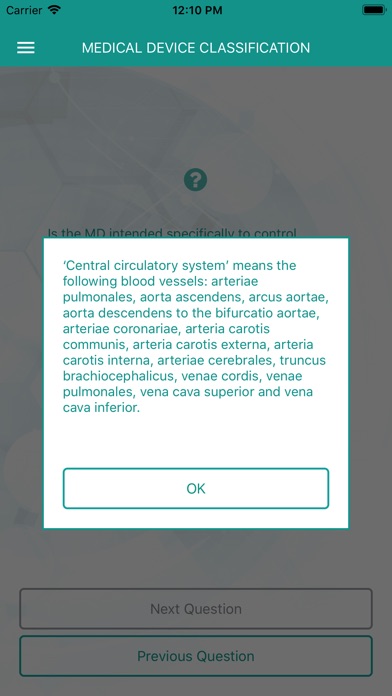
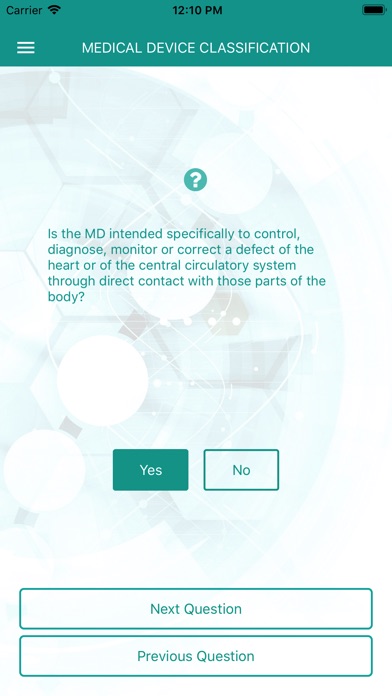
Please answer the key questions and at the end you will see the classification according to MDR 2017/745 for your medical device. For sharing the result with your colleagues and tracing your answers afterwards you can also request a report with the decision tree and the result.
Stop spending money on vague definitions and time consuming considerations. Let us accomplish the tricky part for you and focus on what really counts: a trend-setting medical device.
Do you have anymore questions? Simply contact us: [email protected]